















Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
Bonding in Electron Rich Molecules: Unraveling the Secrets of Chemical Connections

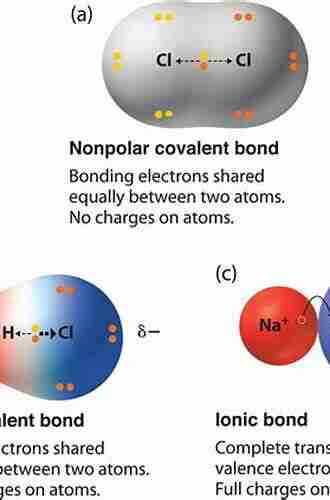
When it comes to chemistry, the formation of bonds between atoms is at the heart of every reaction. These bonds not only dictate the properties and behavior of molecules but also play a crucial role in the functionality of various compounds. Amongst the myriad of bonding types that exist, the bonding in electron-rich molecules stands out as one of the most fascinating and complex avenues of chemical exploration.
So, what exactly are electron-rich molecules? In simple terms, these are molecules that have an excess of electrons, resulting in a negatively charged species. This abundance of electrons creates a unique bonding environment that defies conventional norms and encourages novel chemical interactions.
The Electron-Rich Phenomenon: Manifestations and Manifestations
Electron-rich molecules can arise through a variety of processes, one of which involves the transfer of electrons from a less electronegative atom to a more electronegative one. This process, known as electron donation, can lead to the formation of species such as anionic compounds, radicals, or carbanions. These compounds hold tremendous importance in various chemical fields, including organometallic chemistry, polymer science, and molecular electronics.
4.4 out of 5
| Language | : | English |
| File size | : | 12857 KB |
| Screen Reader | : | Supported |
| Print length | : | 341 pages |
The key to understanding the bonding within electron-rich molecules lies in delving into their molecular orbitals and electron distribution patterns. In traditional bonding scenarios, electrons are shared between atoms to achieve stable molecular structures. However, in electron-rich molecules, the excessive electron density can result in unconventional bonding patterns and unique interactions that challenge classical chemical models.
Electron-Rich Molecules: Breaking the Mold of Bonding
One notable example within electron-rich molecules is the phenomenon of electron sharing between atoms with the same or similar electronegativity. In simple terms, this means that electrons within these molecules tend to occupy molecular orbitals that are centered not just on a single atom, but are distributed over multiple atoms. This sharing of electrons leads to peculiar bonding situations that can foster the formation of exotic compounds and drive novel chemical reactivity.
Furthermore, electron-rich molecules often exhibit a considerable degree of electron delocalization. This means that the electrons within the molecule are not confined to specific regions but instead move freely across the entire structure. This extensive delocalization increases the stability and resilience of the molecule, enabling it to withstand harsh conditions and participate in diverse chemical transformations.
The Ubiquitous Role of Electron-Rich Molecules
The significance of electron-rich molecules extends far beyond theoretical curiosity. These compounds have demonstrated their power and versatility in a multitude of applications. For instance, carbanions, one of the quintessential electron-rich species, serve as essential intermediates in numerous organic synthesis reactions. Their inherent electron-rich nature allows for facile nucleophilic attacks and selective bond formation events, making them invaluable tools for chemists in their quest for novel molecule creation.
Similarly, electron-rich organometallic compounds have found widespread use in catalysis, with transition metals acting as electron reservoirs in such systems. These complexes facilitate a variety of chemical transformations, including cross-coupling reactions, C-H activation, and olefin metathesis. By harnessing the power of electron-rich molecules, chemists can unlock new synthetic routes and enhance the efficiency of existing processes.
Exploring Future Frontiers: Quantum Chemistry and Electron-Rich Molecules
Advancements in quantum chemistry have been instrumental in unraveling the intricacies of bonding within electron-rich molecules. With computational tools becoming increasingly sophisticated, researchers can simulate and analyze the behavior of these compounds with great precision. Quantum calculations allow scientists to explore a wide range of bonding scenarios, predicting the stability and reactivity of electron-rich species under different conditions.
This integration of theory and experimentation has paved the way for designers to develop innovative strategies for the synthesis of electron-rich molecules. By understanding the underlying bonding principles and taking advantage of their unique properties, chemists can design tailor-made compounds with specific applications in mind. From materials science to drug discovery, the possibilities offered by electron-rich molecules are limited only by our imagination.
Embracing the Boundless World of Electron-Rich Molecules
Bonding in electron-rich molecules remains an exciting frontier in chemistry, offering a deep well of knowledge for those willing to explore its depths. These remarkable compounds challenge our understanding of traditional bonding models and open up new avenues for chemical discovery and innovation. By harnessing the unique electronic configurations within electron-rich molecules, scientists have the potential to unearth groundbreaking applications and revolutionize the world we live in.
So, let us embark on this fascinating journey of exploring the intricacies of bonding in electron-rich molecules, unraveling the secrets of chemical connections one electron at a time.
4.4 out of 5
| Language | : | English |
| File size | : | 12857 KB |
| Screen Reader | : | Supported |
| Print length | : | 341 pages |
This second edition was updated to include some of the recent developments, such as “increased-valence” structures for 3-electron-3-centre bonding, benzene, electron conduction and reaction mechanisms, spiral chain O4 polymers and recoupled-pair bonding. The author provides qualitative molecular orbital and valence-bond descriptions of the electronic structures for primarily electron-rich molecules, with strong emphasis given to the valence-bond approach that uses “increased-valence” structures. He describes how “long-bond” Lewis structures as well as standard Lewis structures are incorporated into “increased-valence” structures for electron-rich molecules. “Increased-valence” structures involve more electrons in bonding than do their component Lewis structures, and are used to provide interpretations for molecular electronic structure, bond properties and reactivities. Attention is also given to Pauling “3-electron bonds”, which are usually diatomic components of “increased-valence” structures for electron-rich molecules.

 Samuel Ward
Samuel WardTake Control Of Your Network Marketing Career
Are you tired of working...

 Bryson Hayes
Bryson HayesThe Enigmatic Talent of Rype Jen Selk: A Musical Journey...
When it comes to musical prodigies,...

 Norman Butler
Norman ButlerUnveiling the Rich History and Poetry of Shiraz in...
When it comes to the cultural...

 Cade Simmons
Cade SimmonsHow Impatience Can Be Painful In French And English
: In today's fast-paced world, impatience...

 William Shakespeare
William ShakespeareSewing For Sissy Maids - Unleashing Your Creative Side
Are you ready to dive...

 Harry Hayes
Harry HayesGST Compensation to States: Ensuring Fiscal Stability...
In the wake of the COVID-19 pandemic,...

 Rodney Parker
Rodney ParkerLearn How to Play Blackjack: A Comprehensive Guide for...
Blackjack, also known as twenty-one, is one...

 Wade Cox
Wade CoxComplete Guide Through Belgium And Holland Or Kingdoms Of...
Welcome, travel enthusiasts, to a...

 Jack Butler
Jack Butler15 Eye Popping Projects To Create with Felt Decorations
Felt decorations have become a popular craft...

 Dennis Hayes
Dennis HayesFirst Aid For Teenager Soul Mini Book Charming Petites...
The teenage years can...

 Brett Simmons
Brett SimmonsFrom Fear To Freedom - Overcoming Your Fears and Living a...
Are you tired of living in...

 Carl Walker
Carl WalkerSmoking Ears And Screaming Teeth: The Shocking Truth...
Smoking has long been known to cause a host of...
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Ignacio HayesSoil Science And Management Mark Blitz - Unlocking the Secrets of Earth's...
Ignacio HayesSoil Science And Management Mark Blitz - Unlocking the Secrets of Earth's...
 Matthew WardThe Ultimate Guide to Unleashing Confidence and Empowerment in Women: Beyond...
Matthew WardThe Ultimate Guide to Unleashing Confidence and Empowerment in Women: Beyond...
 Jeff FosterThe Little Rabbit Who Wanted Red Wings: A Heartwarming Tale of Ambition and...
Jeff FosterThe Little Rabbit Who Wanted Red Wings: A Heartwarming Tale of Ambition and... Chandler WardFollow ·16.2k
Chandler WardFollow ·16.2k Neal WardFollow ·12.8k
Neal WardFollow ·12.8k Bruce SnyderFollow ·19.7k
Bruce SnyderFollow ·19.7k Theodore MitchellFollow ·19.8k
Theodore MitchellFollow ·19.8k William WordsworthFollow ·10.1k
William WordsworthFollow ·10.1k Cooper BellFollow ·2.1k
Cooper BellFollow ·2.1k Ricky BellFollow ·12.9k
Ricky BellFollow ·12.9k Samuel BeckettFollow ·7.4k
Samuel BeckettFollow ·7.4k